MEMBRAN FİLTRASYON
Sıvılar birçok farklı molekül veya partikül boyutlarında bileşenlerden oluşur. Sıvıdan ayırmak ve ya konsantre etmek istediğiniz bileşenleri farklı boyutlarda gözeneklere sahip membranları kullanarak tam olarak ayırmak mümkündür.
Membran filtrasyon, sıvıyı yarı geçirgen bir membran kullanılarak iki akışkana ayıran basit bir teknolojidir. Membranlar seçici-geçirgen özellikte olup bir karışımda bazı bileşenlerin geçisine izin verirken diğer bileşenleri tutar. Basınç farkı membran gözenek boyutundan daha küçük olan bileşenleri membrandan geçmeye zorlar ve bu “permeate-süzüntü”dür. Kalan bileşenler ise “retentate-filtre edilmeyen kısım” olarak kalır. Besleme akışı membrana paralel olarak hareket ettiği için proses süresince membran yüzeyinin bloke olması engellenir. Bu işlem çarpraz-akış filtrasyon olarak bilinir.
Spiral sarımlı membran modülünün açık şekli yanda verilmiştir. Ürün taşıyıcı (permeate carrier) iki membran arasına yerleştirilmiştir. İki membran ve ürün taşıyıcı yaprak adı verilen yapıyı oluşturmak için üç kenarı birlikte yapıştırılır. Yapıştırılmamış uçlar merkezdeki delikli topl ama tüpüne bağlanır. Membran yaprağı ağ yapılı besleme ara levhası merkezdeki delikli toplama tüpüne spiral şeklinde sarılır. Yaprak uzunluğunu kısaltmak için birçok membran yaprağı merkez tüpün etrafına aynı anda sarılır. Çoklu yaprak tasarımları ürün akışındaki düşüşü minimize eder. Sarmal modül basınçlı tübüler kap içerisine yerleştirilir.
ÇAPRAZ AKIŞ
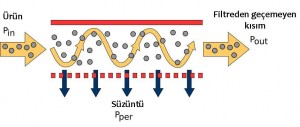 Çapraz akış filtrasyonunda, ürün akışı filtre içinden daha ziyade çoğunlukla filtre yüzeyinin üstünden teğet olarak geçer. Bu akış tipinin prensip olarak avantajı filtreyi tıkayan filtre kekinin filtrasyon süresince yıkanarak filtrenin uzun süre kullanımına olanak sağlar ve sürekli proses olabilir.
Çapraz akış filtrasyonunda, ürün akışı filtre içinden daha ziyade çoğunlukla filtre yüzeyinin üstünden teğet olarak geçer. Bu akış tipinin prensip olarak avantajı filtreyi tıkayan filtre kekinin filtrasyon süresince yıkanarak filtrenin uzun süre kullanımına olanak sağlar ve sürekli proses olabilir.
Bu tip filtrasyon küçük partikül boyutunda katıları yüksek oranda içeren ürünler içindir çünkü katı maddeler klasik filtrasyonda membranı kolayca tıkayabilir. Bu uygulama için fermentasyon sıvısından çözünür antibiyotiklerin ekstraksiyonu endüstriden bir örnektir.
KİRLENME VE KONSANTRASYON POLARİZASYONU
Kirlenme zamanla membranlar arası akışın azalmasına neden olan bir olaydır. Kirlenmeye öncelikle konsantrasyon polarizasyonu neden olur. Konsantrasyon polarizasyonu moleküllerin membran yüzeyinde birikmesidir. Üç tip kirlenme vardır. Bunlar kek tabakası oluşumu, gözeneklerin tıkanması ve gözenek içinin kirlenmesidir. Kek tabakası oluşumu ve gözeneklerin tıkanması membranın yüzeyindeki kirlenmedir.
Kirlenmeye öncelikle konsantrasyon polarizasyonu neden olur. Konsantrasyon polarizasyonu moleküllerin membran yüzeyinde birikmesidir. Üç tip kirlenme vardır. Bunlar kek tabakası oluşumu, gözeneklerin tıkanması ve gözenek içinin kirlenmesidir. Kek tabakası oluşumu ve gözeneklerin tıkanması membranın yüzeyindeki kirlenmedir.
Gözenek tıkanması süresince alıkonan moleküller gözenekleri bloke ederken kek oluşumu sırasında tortulaşmış moleküller membran yüzeyinde birikir. Membran içi gözenek tıkanması membranların gözenekleri arasındaki tıkanmayı açıklar. Moleküller membran gözeneklerinin içinde birikir ve ortalama membran gözenek çapını azaltır. Kirlenmenin üç şekli de çözücünün ve küçük çözünen moleküllerin membran gözeneklerinden taşınmasına karşı koyar.
Genel olarak; yüksek çapraz akış hızı ve düşük işlem basıncı ile en ekonomik çözücü-çözünen ayrımı sağladığı görülmüştür. Yüksek çapraz akış hızları konsantrasyon polarizasyonunu ve kirlenmeyi azaltır. Düşük basınçlarda akış uygulanan basınçla lineer olarak artar. En iyi ayırma için çarpraz akış hızları ve basınç dengesi sağlanmalıdır. Bu denge membran tipi ve besleme çözeltisinin özelliklerine göre değişiklik gösterir.
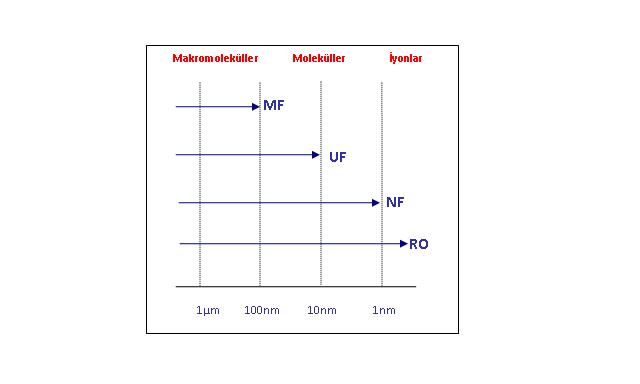
MEMBRAN SEÇİMİ
Membran tipinin doğru seçimi kapasite, verim ve verimlilik açısından optimize edilmiş bir filtrasyon işlemi elde etmek için çok önemlidir. Proses, kullanılan membranın gözenek boyutuna göre Ters Osmoz (RO), Nanofiltrasyon (NF), Ultrafiltrasyon (UF) ve Mikrofiltrasyon (MF) olarak adlandırılır. Membran tipi, tesis tasarımı ve işleme parametreleri, istenen performans ve tüm prosesin maliyet verimliliğini karşılamak için birbiri ile uyumlu olmalıdır.